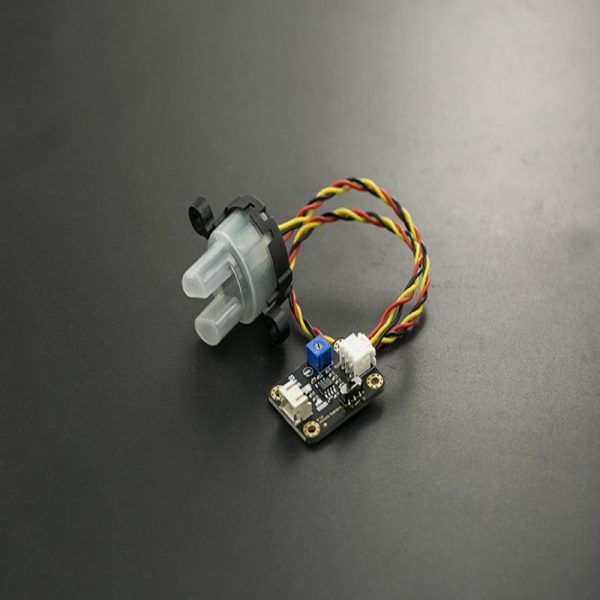
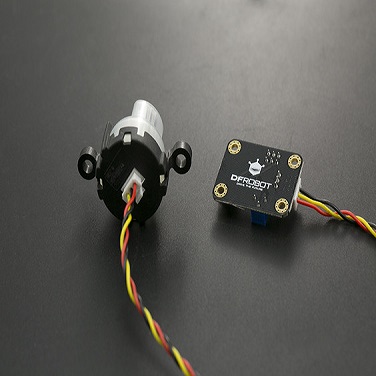
Gravity: Analog Electrical Conductivity Sensor /Meter(K=10) 10~100ms/cm
Gravity: Analog Electrical Conductivity Sensor/Meter(K=10) is particularly used to measure the high electrical conductivity liquid, such as seawater, concentrated brine, etc. The measurement range is up to 100ms/cm. This product is suitable for the water quality application of mariculture, for example, marine fisheries, marine aquariums.

Specifications
Signal conversion table
Power supply voltage: 3.0 ~ 5.0V
Output voltage: 0 ~ 3.2V
Probe Connector: BNC
Signal connector: PH2.0-3Pin
Measurement accuracy: ± 5% F.S.
Panel size: 42mm * 32mm / 1.65in * 1.26in
Electrical conductivity test
Detector type: Laboratory
Fixed cell: 10 ± 2
Support detection range: 10 ~ 100ms / cm
Temperature range: 0 ~ 40 ° C
Probe Life:> 0.5 year (Real life related to frequency of use and scene)
Cable length: 100 ± 2cm
Conductivity is a measure of the ability of water to allow electricity to flow. This ability is directly related to the concentration of ions in the water. These conductive ions are derived from dissolved salts and minerals such as alkalis, chlorides, sulfides and carbonates.
The more ions there are, the higher the conductivity of the water. Distilled or deionized water can act as an insulator due to its very low (if not negligible) conductivity value. Seawater, on the other hand, has a very high conductivity.
Ions produce electricity due to their positive and negative charges. When electrolytes dissolve in water, they are divided into positively charged (cations) and negatively charged (anion) particles. As the solutes are separated in water, the concentrations of each positive and negative charge remain equal. This means that although the conductivity of water increases with the addition of ions, it remains electrically neutral.
Conductivity units
Conductivity is usually measured in micro- or millimeters per centimeter (uS / cm or mS / cm).
Tolerance of aquatic organisms
Most aquatic organisms can only tolerate a certain range of salinity. The physiological adaptation of each species is determined by the salinity of its environment. Most species of fish are exclusively freshwater or exclusively saltwater. However, there are some organisms that can adapt to a range of salinities.
Some aquatic organisms may even be sensitive to the ionic composition of water. The inflow of a particular salt can adversely affect a species, regardless of whether the salinity levels remain within an acceptable range.
Salinity tolerances depend on osmotic processes within an organism. Fish and other aquatic animals that live in fresh water (low conductivity) are hyperosmotic. Hyperosmotic determines a cell’s ability to eliminate water and retain ions. Thus, these organisms maintain higher internal ion concentrations than the surrounding water. At the other end of the spectrum, saline (high-conductivity) organisms are subcellular and maintain a lower internal ionic concentration than seawater. Changing the conductivity of the environment by increasing or decreasing salt levels will adversely affect the metabolic capacity of organisms.
Conduction change may indicate contamination
Oil or hydrocarbons can reduce the conductivity of water.
A sudden increase or decrease in conductivity in a body of water may indicate contamination. Agricultural runoff or sewage leakage will increase conductivity due to additional chloride, phosphate and nitrate ions. Leaking oil or adding other organic compounds will reduce the conductivity as these elements do not break down into ions. In both cases, the added solids will have a negative impact on water quality.


 Botzees
Botzees Keyestudio
Keyestudio Fischertechnik
Fischertechnik