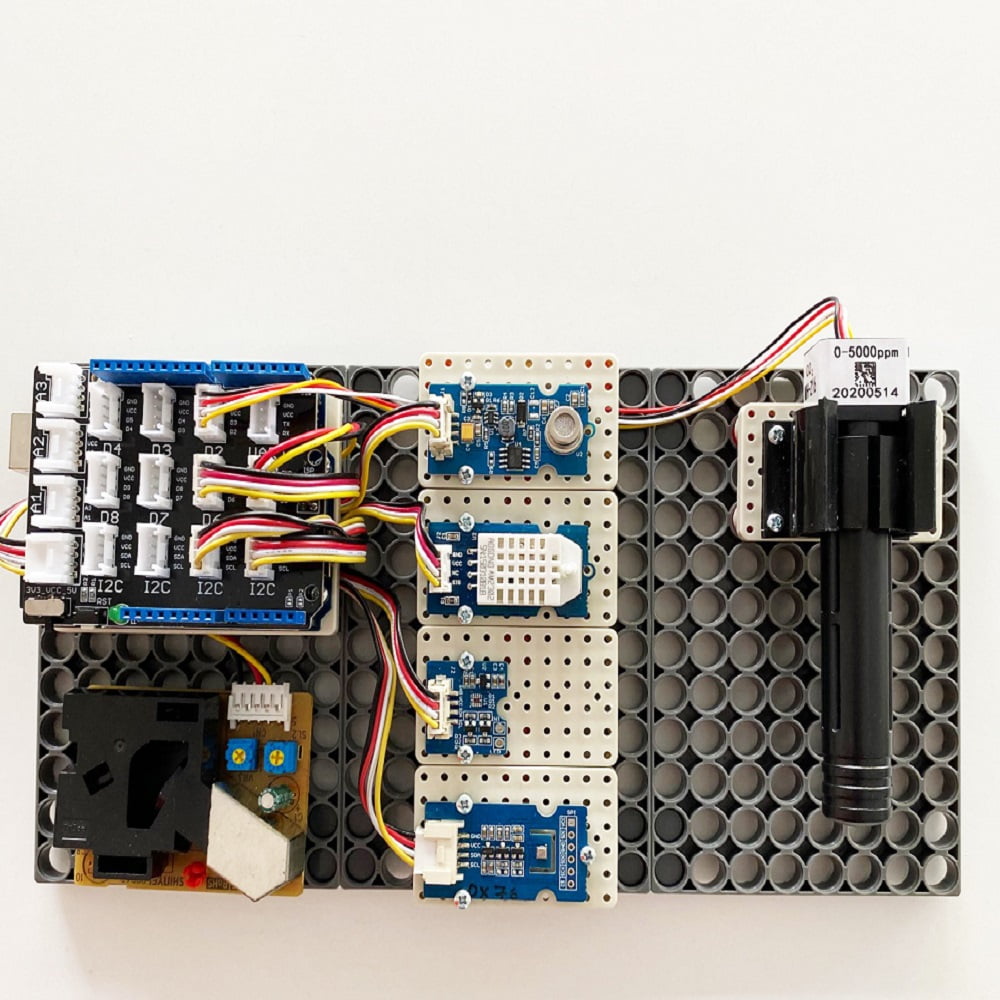
Science School Air Quality IoT
Science School Air Quality IoT is the proposal of Knowledge Research for the “quality of school life” category of the Science School action. It is a fully equipped IoT station for measuring environmental parameters and air quality for the school environment. Contributes to the environmental awareness of children and can be used as educational material in the course of computer science or skills.
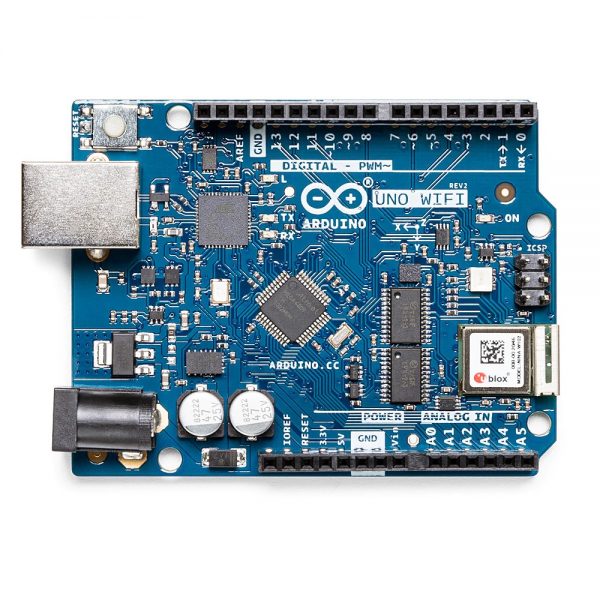
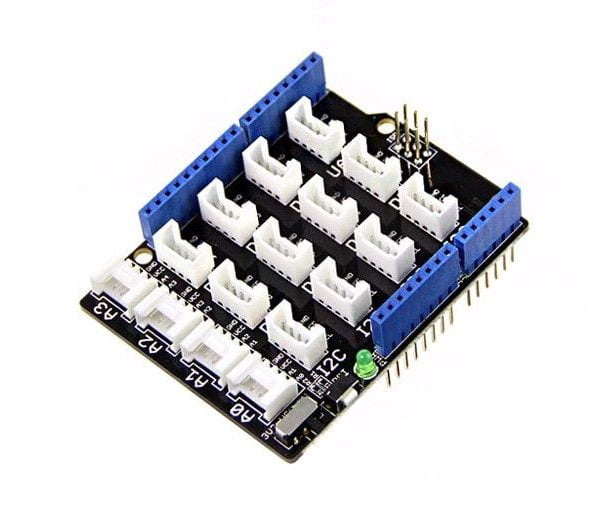
The central unit is the well-known Arduino UNO Wi-Fi which is interfaced with peripheral sensors measuring environmental parameters through the easy-to-use Grove interface system.
The central unit has Wi-Fi communication capability .The measuring station can be easily connected to the school wireless network and communicate its measurements.
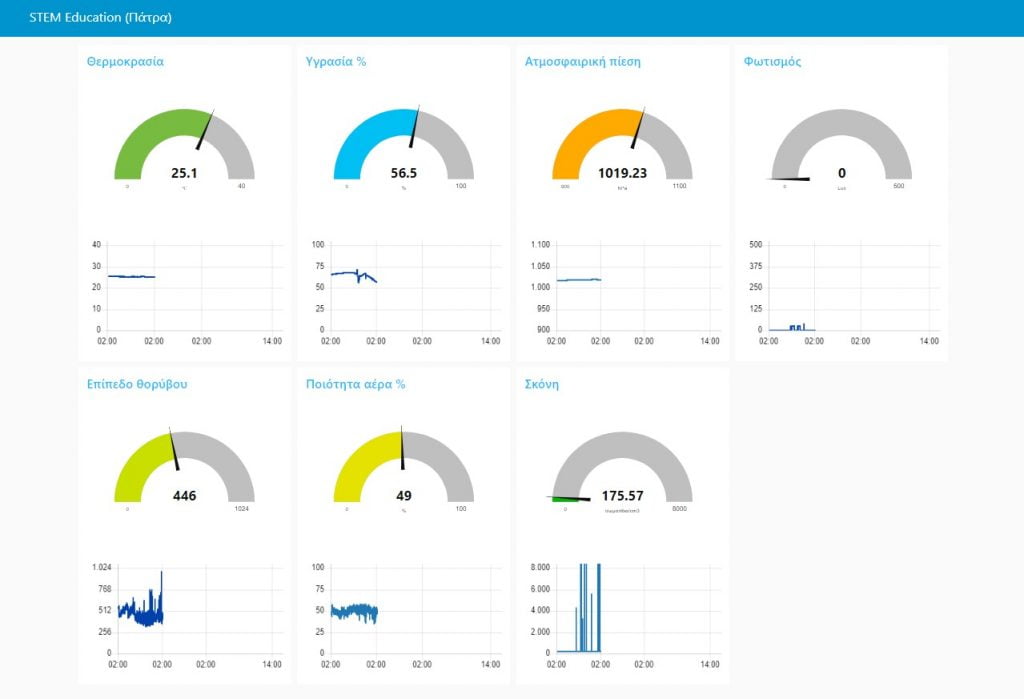
To support the partner stations, a central server for collecting and displaying measurements has been implemented .Through the server the location of the station can be displayed on a map .The measurements of each of its sensors can be displayed in the form of graphs and current value.
A detailed description of the units that make up the Science School Air Quality IoT is presented below:
By Earth’s atmosphere we mean the gaseous body that surrounds the Earth and is held by its gravity, practically reaching an altitude of 3,500 kilometers.
The atmosphere protects life on Earth by absorbing ultraviolet sunlight, heating its surface by retaining heat (a greenhouse effect) and reducing temperature fluctuations between day and night.
Dry air consists of 78.08% of nitrogen, 20.95% of oxygen, 0.93% of argon, 0.0395% of carbon dioxide and traces of other gases.

Air pollution
Air pollution is the presence of substances in the atmosphere that are harmful to the health of humans and other living things or cause damage to the climate or materials. There are different types of air pollutants, such as gases , particles (both organic and inorganic) and biological molecules. Air pollution can cause disease, allergies and even death in humans. It can also cause damage to other living organisms, such as animals and food crops, and can damage the natural or structured environment. Both human activity and natural processes can create air pollution.

ompounds (VOCs) – VOCs are a well-known external air pollutant. They are categorized as either methane (CH4) or non-methane (NMVOCs). Methane is a highly efficient greenhouse gas that helps increase global warming. Other hydrocarbon VOCs are also important greenhouse gases due to their role in creating ozone . Hydrocarbon VOCs can alson extend the life of methane in the atmosphere.
Chlorofluorocarbons (CFCs) – harmful to the ozone layer. Emitted from products is prohibited for use. These are gases released by air conditioners, refrigerators, aerosol sprays, etc . Upon release into the air, CFCs ascend into the stratosphere. Here they come in contact with other gases and destroy the ozone layer. This allows harmful UV rays to reach the earth’s surface.
Persistent organic pollutants (POPs) are organic compounds that are resistant to environmental degradation through chemical, biological and photolytic processes. Because of this, they have been observed to remain in the environment . POPs have the ability to travel long distances , bioaccumulate in human and animal tissues .Also they can biomagnetize food chains . They can have potentially significant effects on human health and the environment.
Secondary pollutants include:
Smog is a type of air pollution. Classic smog results from large amounts of carbon burning in an area caused by a mixture of smoke and sulfur dioxide. Modern smog is not usually derived from carbon, but from emissions from vehicles and industries . These are activated into the atmosphere by ultraviolet light from the sun to form secondary pollutants that are also combined with primary emissions to form photochemical smog.
Soil ozone (O3) formed by NOx and VOC. Ozone (O3) is a key component of the troposphere. It is also an important component of certain areas of the stratosphere commonly known as the ozone layer. The photochemical and chemical reactions involved involve many of the chemical processes that take place in the atmosphere day and night. At unusually high concentrations caused by human activities (mainly the burning of fossil fuels), it is a pollutant and a component of smog.
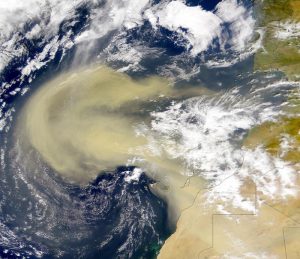
Atmospheric or wind-borne dust, also known as wind dust .It comes from arid areas where high-speed winds are able to remove mainly mud-sized material from sensitive surfaces. This includes areas where grazing, plowing, vehicle use and other human activities have further destabilized the land. One third of the world’s land is covered by dust . Some super-arid regions such is the Sahara, which covers 0.9 billion hectares, and arid land, which covers 5.2 billion hectares.
How Dust is produced?
Dust in the atmosphere is produced by salting and sandblasting of grain-sized sand and is transported through the troposphere. This airborne dust is considered an aerosol and once in the atmosphere, it can produce strong local radiation. Sahara dust in particular can be transported and deposited to the Caribbean and Amazon basins . This can affect air temperatures, cause ocean cooling and alter rainfall.
A location that combines an average temperature of 19 degrees Celsius, 60% average humidity and a temperature range of about 10 degrees Celsius around the average temperature (annual temperature change) is considered ideal for the comfort of the human species. Most places with these characteristics are found during the transition from temperate and tropical climates, around the tropics, especially in the southern hemisphere (the tropical Capricorn)
What is Atmospheric Pressure?
Atmospheric pressure, also known as barometric pressure (after the barometer), is the pressure in the Earth’s atmosphere. The typical atmosphere (symbol: atm) is a unit of pressure defined as 101,325 Pa (1,013.25 hPa, 1,013.25 mbar), which is equivalent to 760 mm Hg, 29.9212 inches Hg or 14,696 psi. The unit atm is approximately equivalent to the average atmospheric pressure of sea level on Earth, that is, the atmospheric pressure of the Earth at sea level is about 1 atm.
On their way into the atmosphere, the long wavelengths of light (red, orange) manage to cross it without much impact, while those with the shortest wavelengths (purple, blue) are scattered in all directions by the air molecules. Thus, the blue color of the spectrum reaches our eyes from all directions. Of course, we would expect the color of the sky to be purple and not blue, as it has a shorter wavelength.
But this does not happen for three reasons :
a) purple has a low intensity compared to other colors in the spectrum b) the eyes are more “sensitive” to the three basic colors (red, blue, green) and c) most of it purple is absorbed into the upper atmosphere and does not manage to reach the surface of the Earth. Finally, at night when there is no sunlight this phenomenon does not happen (Rayleigh scattering) and the sky is black.
Looking at the horizon the color of the sky begins to fade?
Many times one can observe looking at the horizon, that the color of the sky begins to fade from blue and tend to white. This is because the light from the horizon has a longer path to reach us. Thus, it has been absorbed and dispersed more than once. The Earth also plays the role of scattering and resurrecting this light. As a result of this increased scattering, the dominance of blue decreases.
Sunset and Sunrise Colors
When the Sun sets or rises the color of the sky has an orange-red color. This is because the solar disk is very low on the horizon, so its light will need to travel longer lengths of the atmosphere to reach the observer. Near the surface the air is thicker and there are more dust particles, pollutants and mist droplets. Then the short wavelengths (purple, blue) are completely scattered, due to scattering and do not reach the eyes of the observer. In contrast, long wavelengths (red, orange) manage to “survive” and for this reason the solar disk acquires the color red.


 Botzees
Botzees Keyestudio
Keyestudio Fischertechnik
Fischertechnik